Julia is a Brazilian child with an ultra-rare disease called Neuronal Ceroid Lipofuscinosis, type 7 (CLN7). Treatment for this disease is carried out in the USA and costs 18 million reais. We need your donation to give Julia a new chance at life!
Ceroid Lipofuscinosis Neuronal Type 7 (CLN7)
The Fight Against CLN7
We’re in a race against time to save the life of 4-year-old Julia Pontes Domingues, diagnosed with CLN7, a rare degenerative disease.
Our goal is to raise $3 Million to fund Phase 3 of a clinical trial in the US and ensure that Julia and other children with CLN7 have access to the drug.
*Donation amount received based on the exchange rate of USD to BRL at BRL 5.35.
Options to Help

Card or PayPal

GoFundMe

Donation Transfer
Who's Julia?
Julia is from São Domingos do Prata, Minas Gerais, Brazil. The daughter of Fernanda Pontes and Alan Domingues, she is full of life, loving and smiling. Ever since she was little, she has always been smart and active, but over time some signs began to appear that worried her family: difficulties with balance, speech, motor coordination and cognition.


After months of consultations and tests, the difficult confirmation came: Julia has CLN7 (Ceroid Lipofuscinosis Neuronal Type 7), a rare degenerative disease which, without treatment, progressively compromises her movements, speech, vision and cognition.
Now, her family is fighting against time to secure the treatment that can stop this disease and give Julia the chance of a future.

Proof of Diagnosis
Whole Exome Sequencing
The Whole Exome sequencing test confirmed the mutation in the MFSD8 gene, responsible for CLN7 (Ceroid Lipofuscinosis Neuronal Type 7). This genetic report is the main scientific evidence of the diagnosis, validating Julia’s condition and the urgent need for the medication.
This test is essential to ensure the accuracy of the diagnosis and to enable Julia to be included in the clinical study.
Proof of Diagnosis
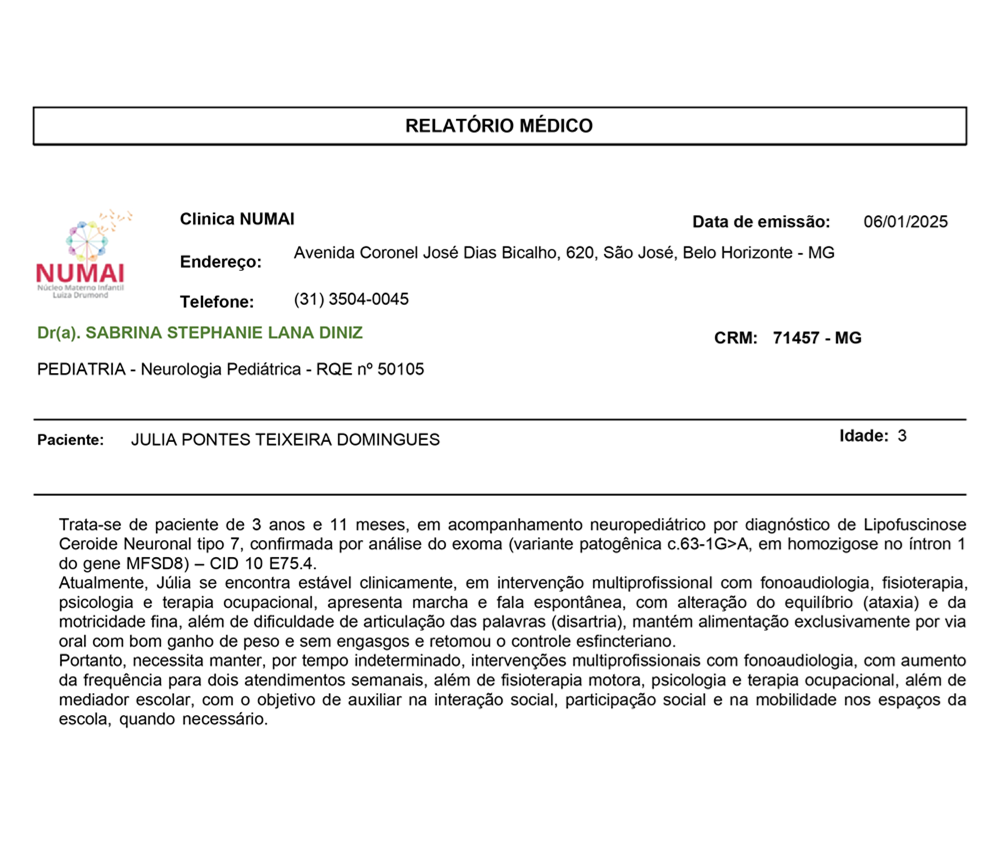
Medical Report
This report issued by the doctor in charge confirms Julia’s diagnosis of CLN7 (Ceroid Lipofuscinosis Neuronal Type 7), proven by genetic analysis of the whole exome sequencing.
It states the current clinical state and the urgency of treatment, with the ongoing need for these interventions to help with mobility, speech and social interaction. However, without adequate treatment, the progression of the disease can compromise their quality of life.
Credibility and Recognition
Elpida Therapeutics
Elpida Therapeutics (California, USA), in partnership with the University of Texas Southwestern Medical Center, has developed a promising experimental drug for CLN7. This drug could halt the progression of the disease, offering a real chance at life for Julia and other children.

Credibility and Recognition
Elpida Therapeutics
An article in Global Genes shows that gene therapy for ultra-rare diseases is advancing with the innovative launch of Elpida Therapeutics SPC.

Letter of Commitment
Elpida Therapeutics
Elpida Therapeutics hereby formalizes its partnership with the Save Julia campaign, confirming its commitment to continuing CLN7’s research and including Julia in the study as soon as the necessary funds are raised.
This document includes:
✅ Confirmation of the collaboration between Elpida and Julia’s family.
✅ Details of the costs of continuing Phase 3.
✅ The transparency and seriousness of the commitment to the campaign.
Evidence
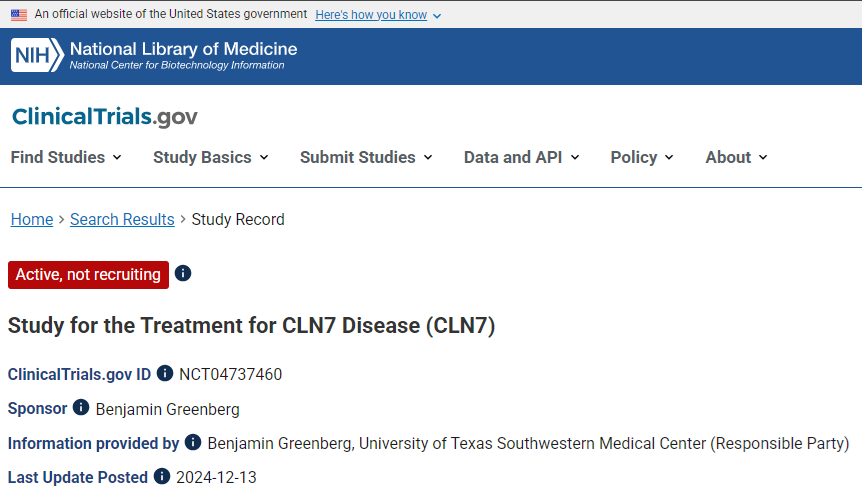
Clinical Study in Progress
The treatment for CLN7 is already in development and officially registered on ClinicalTrials.gov, one of the world’s largest clinical trial databases, being conducted by the team of researchers at the Southwestern Medical Center at the University of Texas.
Evidence
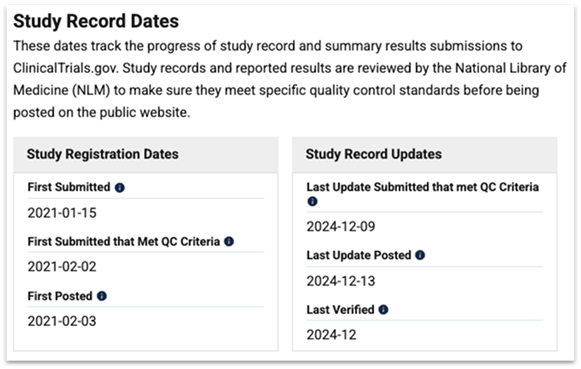
FDA Regulation and Supervision
This section of the ClinicalTrials.gov website provides information on clinical trial regulation and supervision by the FDA (Food and Drug Administration).
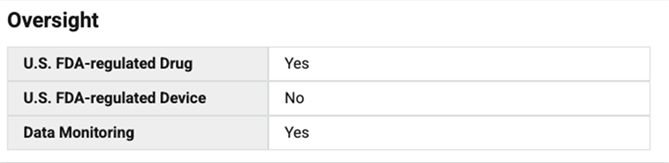
- U.S. FDA-regulated Drug: Yes (The study involves a drug regulated by the FDA, requiring approval before it can be used in patients)
- U.S. FDA-regulated Device: No (The study does not involve medical devices, only a drug)
- Data Monitoring: Yes (There is a monitoring system in place to ensure the safety and accuracy of the data collected in the study)
Evidence
FDA Regulation and Supervision
This section of the ClinicalTrials.gov website provides information on clinical trial regulation and supervision by the FDA (Food and Drug Administration).

Evidence
Update (BDSRA Foundation)
The Batten Disease Support and Research Association (BDSRA Foundation) published an update on January 18, 2024, regarding advances in CLN7 (Batten Disease) research, reinforcing the scientific community’s commitment to finding an effective treatment.
The event highlighted on the screen brought together experts and researchers working to develop solutions for this rare and devastating disease. This type of initiative validates the seriousness of the study and the importance of funding to achieve further advancements.
